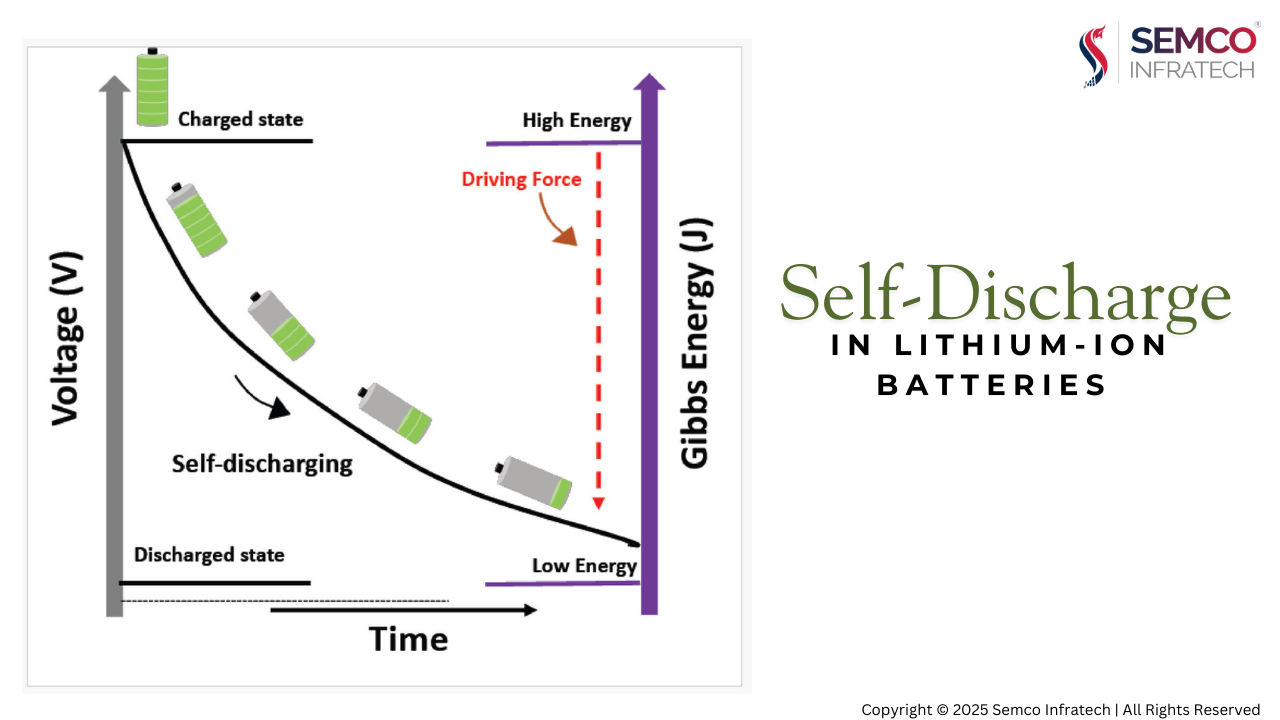
Lithium-ion batteries, despite their high energy density, exhibit a gradual loss of charge even when not in use. This phenomenon, known as self-discharge, significantly impacts battery lifespan and performance. Understanding the underlying mechanisms of self-discharge is crucial for optimizing battery design and maximizing operational life.
Defining Self-Discharge
Self-discharge refers to the spontaneous loss of battery capacity while in an open-circuit state. This capacity loss can be categorized as:
- Reversible Self-Discharge: This portion of capacity loss can be recovered through recharging. It primarily stems from physical factors within the battery.
- Irreversible Self-Discharge: This portion of capacity loss cannot be recovered through recharging. It is primarily attributed to irreversible chemical reactions occurring within the battery.
Quantifying Self-Discharge: The K-Value
The “K-value” is a crucial parameter used to quantify the self-discharge rate of a lithium-ion battery. It represents the voltage drop per unit of time under specific conditions (e.g., high temperature or room temperature). A lower K-value generally indicates better battery performance.

Calculation:
K-value = (OCV1 – OCV2) / (t1 – t2)
- OCV1: Open Circuit Voltage at time t1
- OCV2: Open Circuit Voltage at time t2
Significance:
The K-value helps identify batteries with high self-discharge rates, enabling manufacturers to screen out defective units during production.
Factors Contributing to Self-Discharge
Physical Micro-Short Circuits:
- Dust and Foreign Matter: Particles or debris can bridge the gap between the positive and negative electrodes, creating a direct current path and causing a continuous discharge.
- Diaphragm Misalignment/Damage: The separator, designed to isolate the electrodes, can be misaligned or damaged during manufacturing, leading to direct contact and short circuits.
- Metal Impurities: Metal impurities on the electrodes or within the separator can create micro-short circuits. For example, metal particles can pierce the separator, directly connecting the electrodes.
- External Electronic Leakage: Poor insulation of the battery seal, gasket, or external leads can lead to current leakage, contributing to self-discharge.
Chemical Side Reactions:
- Moisture: Moisture within the battery can react with the electrolyte (often containing lithium hexafluorophosphate (LiPF6)) to generate hydrofluoric acid (HF). HF can damage the Solid Electrolyte Interphase (SEI) layer, leading to electrolyte decomposition, active lithium consumption, and reduced capacity.
- Electrolyte Decomposition: Certain components within the electrolyte may decompose under high voltage conditions, leading to the formation of byproducts that consume active lithium and reduce battery capacity.
Electrode Material Degradation:
- Positive Electrode: At high states of charge, the positive electrode material can react with the electrolyte, leading to the formation of byproducts and capacity loss.
- Negative Electrode: Metal impurities within the negative electrode can react with the conductive carbon, forming a galvanic cell. This can lead to the dissolution of metal ions into the electrolyte, their migration to the negative electrode, and the formation of metal dendrites. These dendrites can grow and eventually pierce the separator, causing a short circuit.
Minimizing Self-Discharge:
- Strict Quality Control: Minimize the presence of foreign particles, ensure proper alignment of the separator, and employ rigorous quality control measures during battery manufacturing.
- Optimize Electrolyte Composition: Utilize high-purity electrolytes with improved stability and resistance to side reactions.
- Improve Electrode Material Quality: Minimize the presence of impurities within the electrode materials.
- Optimize Storage Conditions: Store batteries in cool, dry environments to minimize side reactions and reduce the impact of temperature on self-discharge rates.
By understanding the underlying mechanisms of self-discharge and implementing strategies to mitigate these factors, manufacturers can significantly improve the lifespan and performance of lithium-ion batteries, enhancing their overall value and reliability.